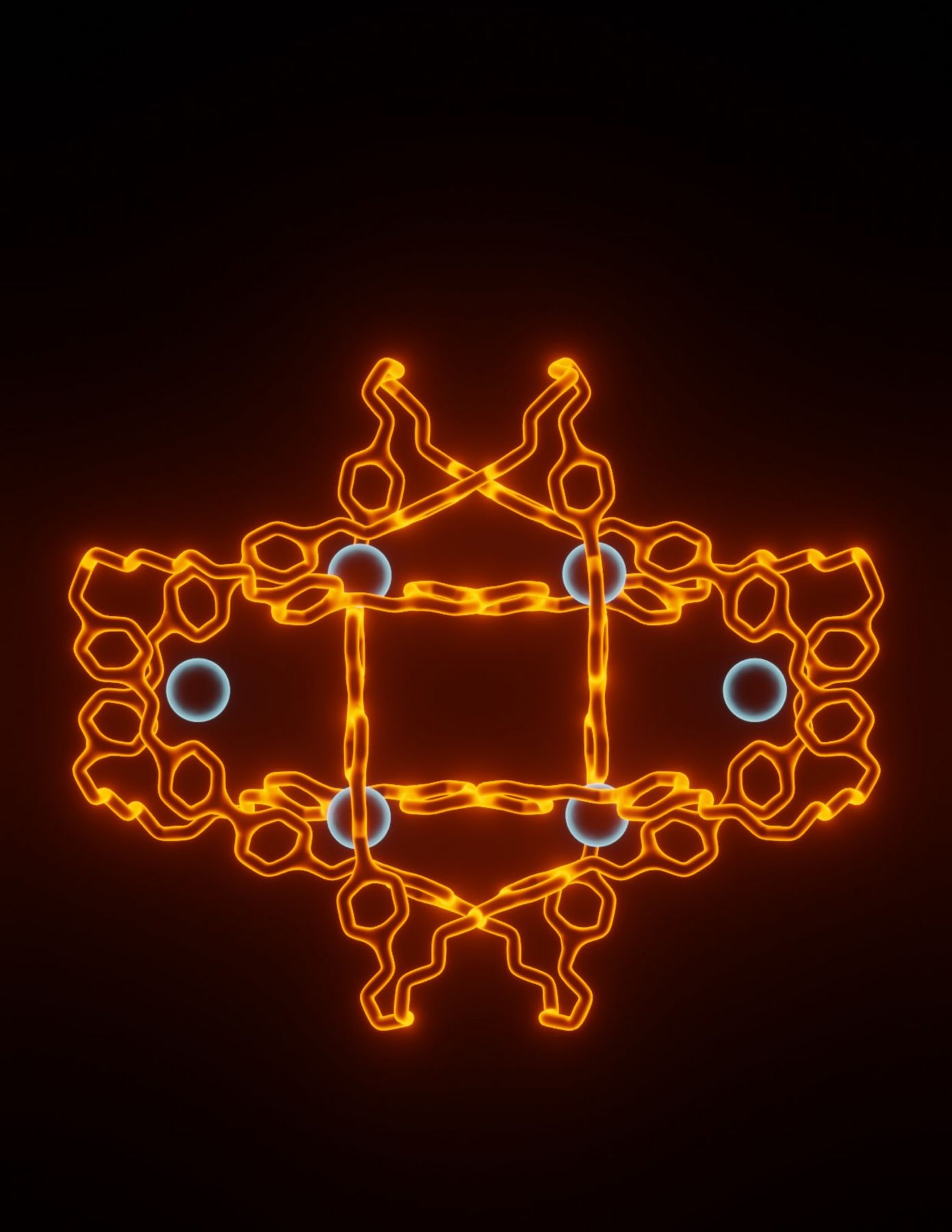
Knots are ubiquitous in the macroscopic world, with uses ranging from tying shoelaces to fishing, and they transform the properties of the thing that is tied. Knots are similarly found at the nanoscale: for example, in proteins and DNA, and also in man-made polymers. These molecular entanglements, likewise, control the properties of the molecules in which they are tied—chain breakage in polymers occurs most readily at the knotted point, and knotting in biomolecules can enhance stability. Further, the knotting of biomolecules impacts their function and enables them to carry out new tasks. Likewise, complex topologies underpin the operation of many synthetic molecular machines. The ability to generate and control more complex architectures is essential to endow these machines with more advanced functions.
Here, we report the synthesis of a molecular knot with eight crossing points, consisting of a single organic loop woven about six templating metal centers, via one-pot self-assembly from a pair of simple dialdehyde and diamine subcomponents and a single metal salt. As the 819 knot that we form is intrinsically topologically chiral, we show that we can diastereoselectively form the knot by using an enantiopure building block, allowing us to use point chirality to control the topological chirality of this complex architecture. The structure and topology of the knot were established by NMR spectroscopy, mass spectrometry, and X-ray crystallography. Upon demetallation, the purely organic strand relaxes into a symmetric conformation, while retaining the topology of the original knot. This knot is topologically chiral and may be synthesized diastereoselectively through the use of an enantiopure diamine building block.
Download a copy of the manuscript (preprint version)