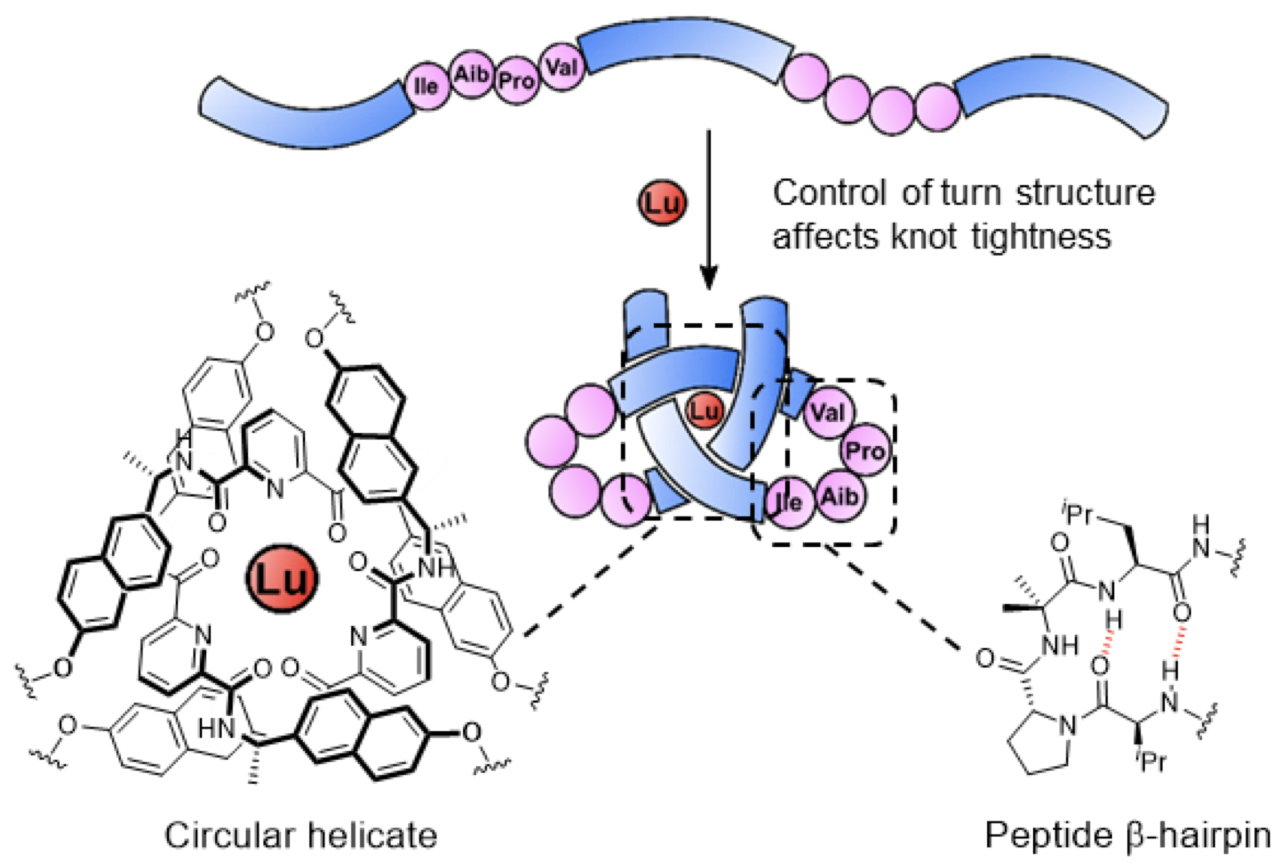
The length and constitution of spacers linking three 2,6-pyridinedicarboxamide units in a molecular strand influence the tightness of the resulting overhand (open-trefoil) knot that the strand folds into in the presence of lanthanide(III) ions. The use of β-hairpin forming motifs as linkers enables a metal-coordinated pseudopeptide with a knotted tertiary structure to be generated. The resulting pseudopeptide knot has one of the highest backbone-to-crossing ratios (BCR)—a measure of knot tightness (a high value corresponding to looseness)—for a synthetic molecular knot to date. Preorganization in the crossing-free turn section of the knot affects aromatic stacking interactions close to the crossing region. The metal-coordinated pseudopeptide knot is compared to overhand knots with other linkers of varying tightness and turn preorganization, and the entangled architectures characterized by NMR spectroscopy, ESI-MS, CD spectroscopy and, in one case, X-ray crystallography. The results show how it is possible to program specific conformational properties into different key regions of synthetic molecular knots, opening the way to systems where knotting can be systematically incorporated into peptide-like chains through design.
Download “Article” Effects_turnstructure_folding_molecular_knots.pdf – Downloaded 359 times – 1 MB
Download a copy of the manuscript