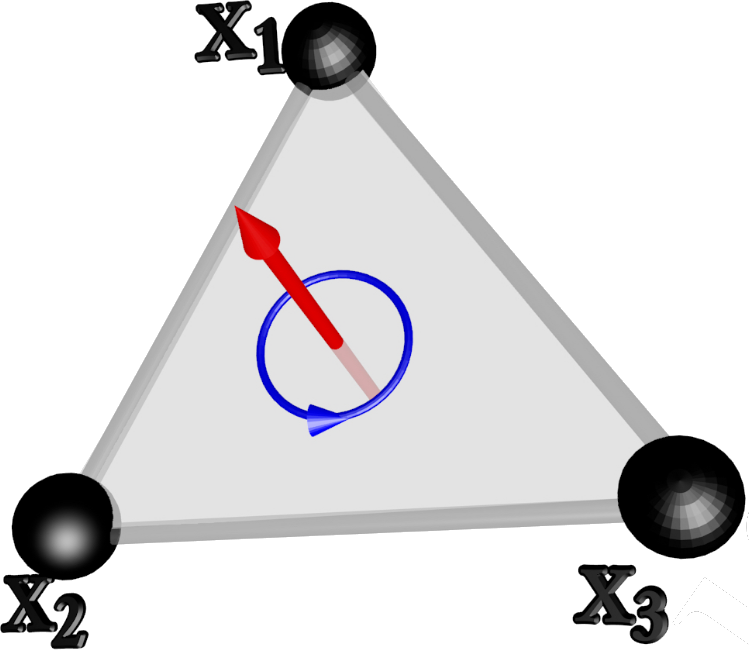
A deformable body can rotate even with no angular momentum simply by changing its shape. Here the first all-atom level molecular dynamics example of this phenomenon is presented. For this the thermal vibrations of individual atoms in an isolated cyclopropane molecule are simulated in vacuum and at ultralow internal temperature values. When the molecule is observed stroboscopically, at discrete equidistant time steps, the random thermal vibrations of the individual atoms become self-organized into a collective oscillatory motion of the entire molecule. The period of oscillation is emergent and intrinsic to the molecule so that this self-organization bears resemblance to a driven time crystal. The oscillation period increases in a self-similar manner when the length of the stroboscopic time step is increased. In the limit of very long stroboscopic time steps the entire molecule can then rotate in an apparent uniform fashion, but with no angular momentum. It is proposed that the observed behavior is universal in the case of triangular molecules. Moreover, it is shown that the emergent uniform rotation without any angular momentum, can be described in an effective theory approach as an autonomous Hamiltonian time crystal. The emergent oscillatory motion appears to be highly sensitive to temperature. This proposes that potential applications could be found from the development of molecular level detector to sensor and control technologies.
Download “Article preprint” Rotation_by_deformation.pdf – Downloaded 241 times – 2 MB
Download a copy of the manuscript