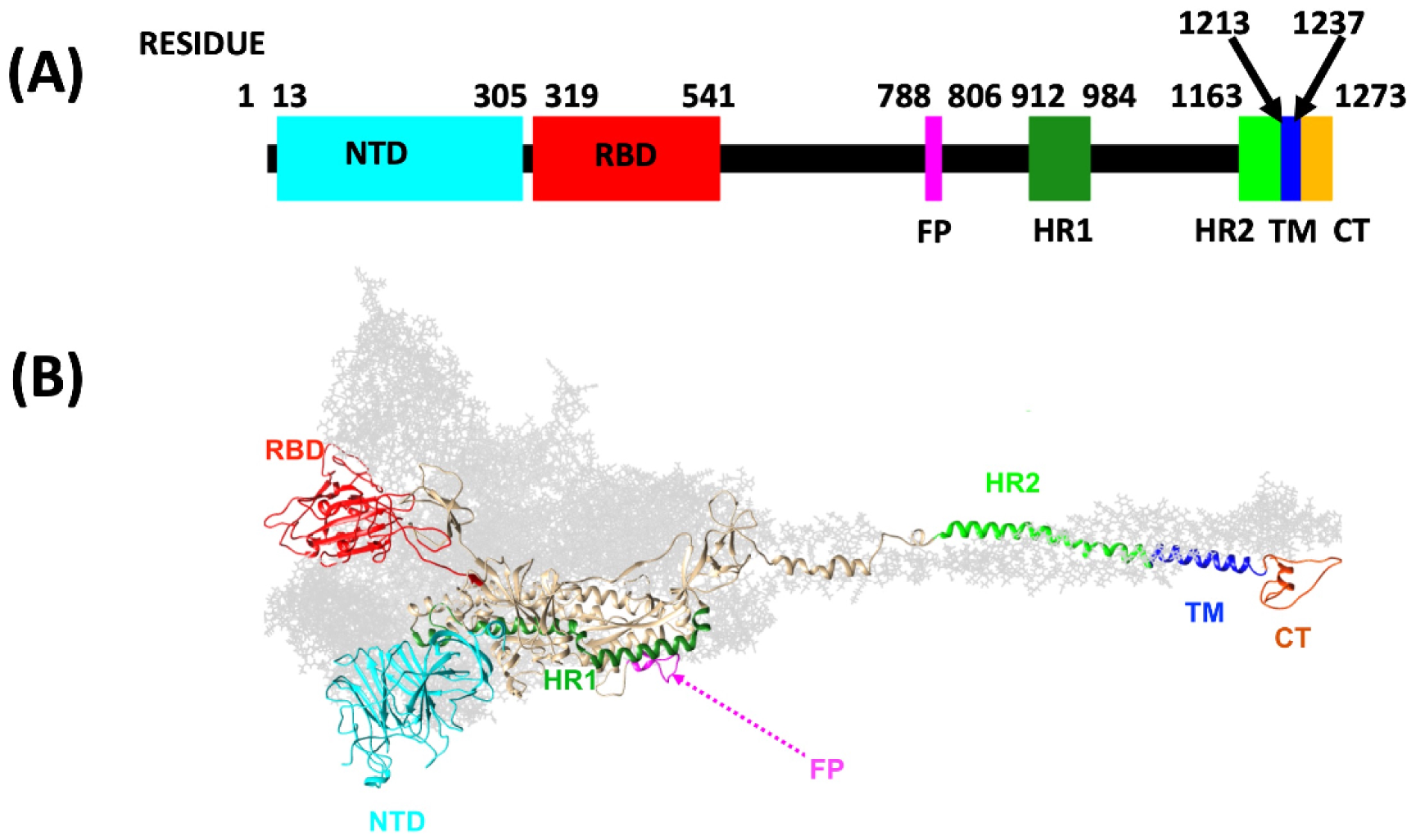
Novel topological methods are introduced to protein research. The aim is to identify hot-spot sites where a bifurcation can change the local topology of the protein backbone. Since the shape of a protein is intimately related to its biological function, a mutation that takes place at such a bifurcation hot-spot has an enhanced capacity to change the protein’s biological function. The methodology applies to any protein but it is developed with the SARS-CoV-2 spike protein as a timely example. First, topological criteria are introduced to identify and classify potential mutation hot-spot sites along the protein backbone. Then, the expected outcome of a substitution mutation is estimated for a general class of hot-spots, by a comparative analysis of the backbone segments that surround the hot-spot sites. This analysis employs the statistics of commensurable amino acid fragments in the Protein Data Bank, in combination with general stereochemical considerations. It is observed that the notorious D614G substitution of the spike protein is a good example of such a mutation hot-spot. Several topologically similar examples are then analyzed in detail, some of them are even better candidates for a mutation hot-spot than D614G. The local topology of the recently observed N501Y mutation is also inspected, and it is found that this site is prone to a different kind of local topology changing bifurcation.
Download a copy of the manuscript (preprint version)