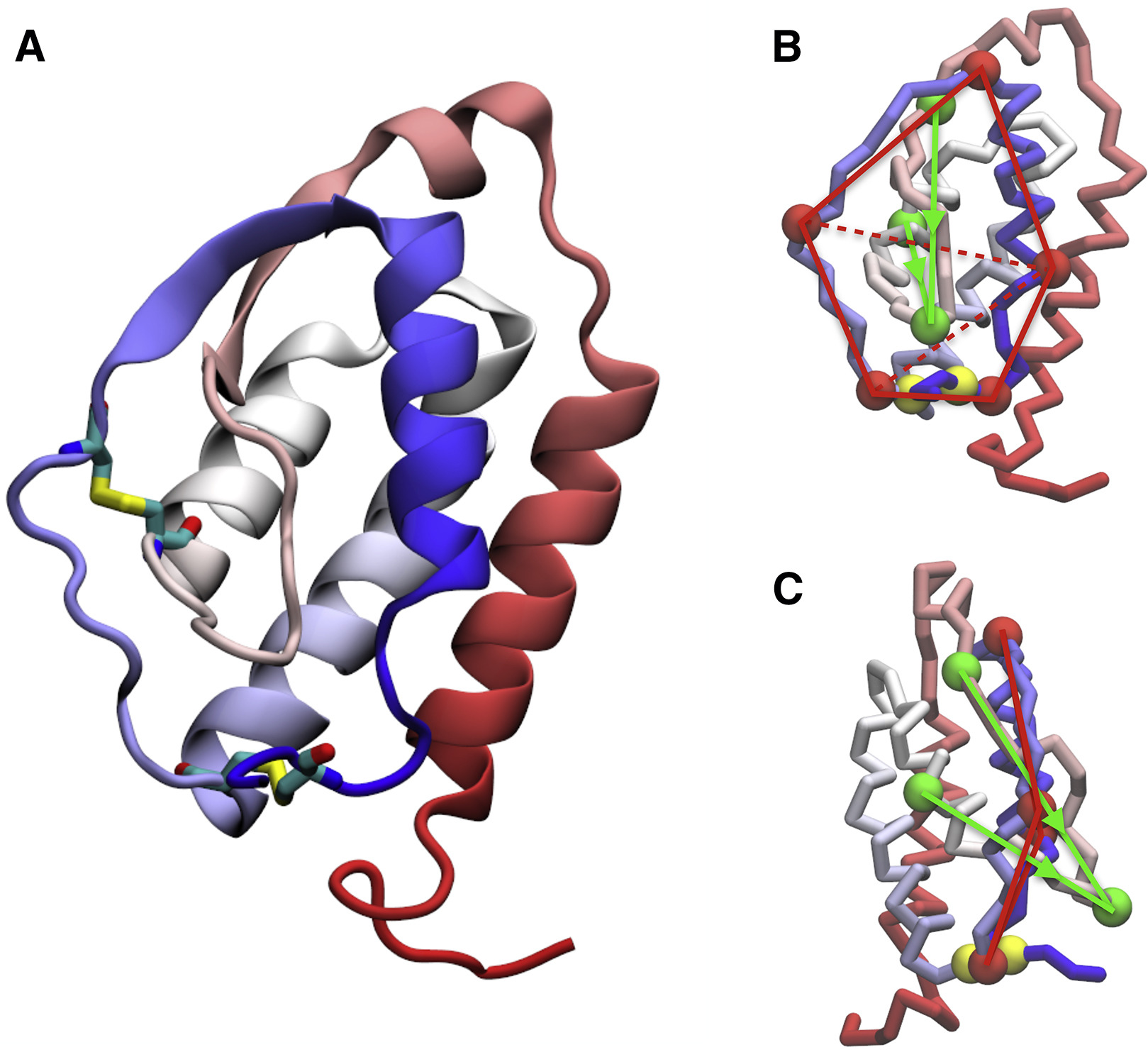
We have investigated the folding mechanism of granulocyte-macrophage colony-stimulating factor, a glycoprotein that handles diverse functions in the human body. This protein folds in a rather common self-entangled conformation named complex lasso. Understanding how a polypeptide encodes into its sequence the capability of tying itself into such kinds of self-entangled structures would represent a major advancement in the comprehension of protein
folding. To study this folding mechanism, we have employed molecular dynamics simulations, using both a well-known minimalistic model of the protein and an alternative model specifically designed to highlight the preferential pathways of entangled folding. Our calculations show how the protein can avoid the kinetic traps related to self-entanglement, managing to fold in a reproducible and efficient way.
Download “Article” Searching_the_Optimal_Folding.pdf – Downloaded 301 times – 6 MB
Download a copy of the manuscript