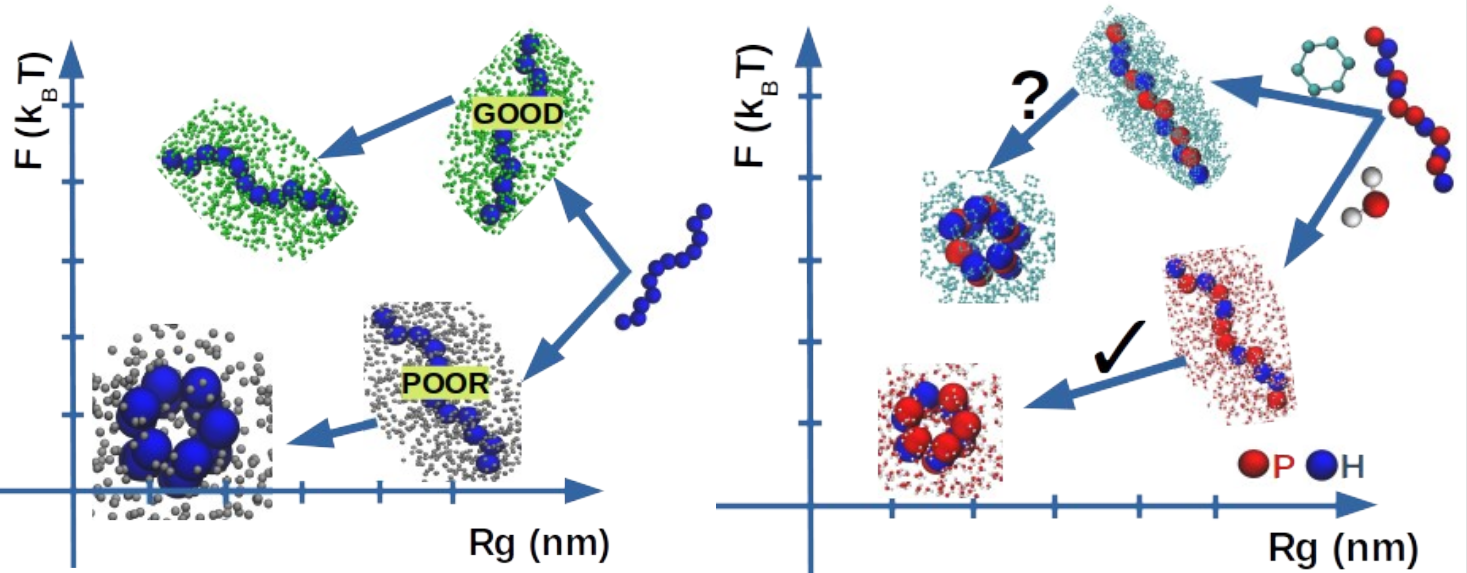
Using molecular dynamics and thermodynamic integration, we report on the solvation process in water and in cyclohexane of seven polypeptides (GLY, ALA, ILE, ASN, LYS, ARG, GLU). The polypeptides are selected to cover the full hydrophobic scale while varying their chain length from tri- to undeca-homopeptides provide indications on possible non-additivity effects as well as the role of the peptide backbone in the overall stability of the polypeptides. The use of different solvents and different polypeptides allows us to investigate the relation between solvent quality — the capacity of a given solvent to fold a given biopolymer often described on a scale ranging from “good” to “poor”, and solvent polarity — related to the specific interactions of any solvent with respect to a reference solvent. Hydrophobic polypeptides are found to collapse in water (polar solvent) and to keep an extended conformation in cyclohexane (apolar solvent) thus supporting the usual view of “like-dissolves-like”. By contrast, polar polypeptides present a more complex behavior defying this rule. All considered polypeptides are found to have a favorable solvation free energy independently to the solvent polarity and their intrinsic hydrophobicity, clearly highlighting the prominent stabilizing role of the peptide backbone, with the solvation process largely enthalpically dominated in polar polypeptides and mostly entropically driven for hydrophobic polypeptides. The absence of a mirror symmetry upon the inversion of polarities of both the solvent and the polypeptides is confirmed.
Download “Article” Solvent_quality_and_solvent_polarity.pdf – Downloaded 205 times – 6 MB
Download a copy of the manuscript