Status
Completed
Period
1-16 September 2022
Applicant
Simone Adorinni
Home Institution
Dept. of Chemical & Pharmaceutical Sciences, University of Trieste, Italy
Host Contact
Prof. Jonathan Nitschke
Host Institution
Yusuf Hamied Department of Chemistry, United Kingdom
Aim of the mission
The aim of the project is the synthesis of new metal-organic cages (MOCs) having a gelator peptide as external ligand and able to form gels. In a recent study, an iron MOC containing a tripeptide a tri-aldehyde has been completely characterised. This cage is able to form a gel at a concentration 5 mM. To prove the generality of the approach, new MOCs, differing only for one of the subcomponents (the peptide and the metal salt), have already been confirmed by the NMR and MS spectroscopies. The missing step is the synthesis of new cages that differ for the last subcomponent, the aldehyde. The goal of the proposal is the synthesis of new aldehydes that will be subsequently tested for the formation of new cages and gels.
Professor Nitschke is a world-leader in the study of metal-organic coordination cages as evidenced by his outstanding publications’ record, in top journals such as Nature, Science, Nature Chemistry, Advanced Materials, Journal of the American Chemical Society, and Angewandte Chemie. In his group they use chemical self-assembly to create complex structures with targeted functions from simple building blocks. Their work deals with the preparation of complex structures using a process we refer to as subcomponent self-assembly. The selective guest encapsulation properties of these metal organic cages have been used to trap and stabilize unstable species, separate substrates as diverse as gases and fullerenes, and as catalysts and sensors.
Summary of the Results
Three aldehydes were successfully synthesised and fully characterised through NMR and mass spectroscopies during this STSM. During the mission the different aldehydes has been tested for the formation of three new cages, that differ from the previous ones in several aspects.
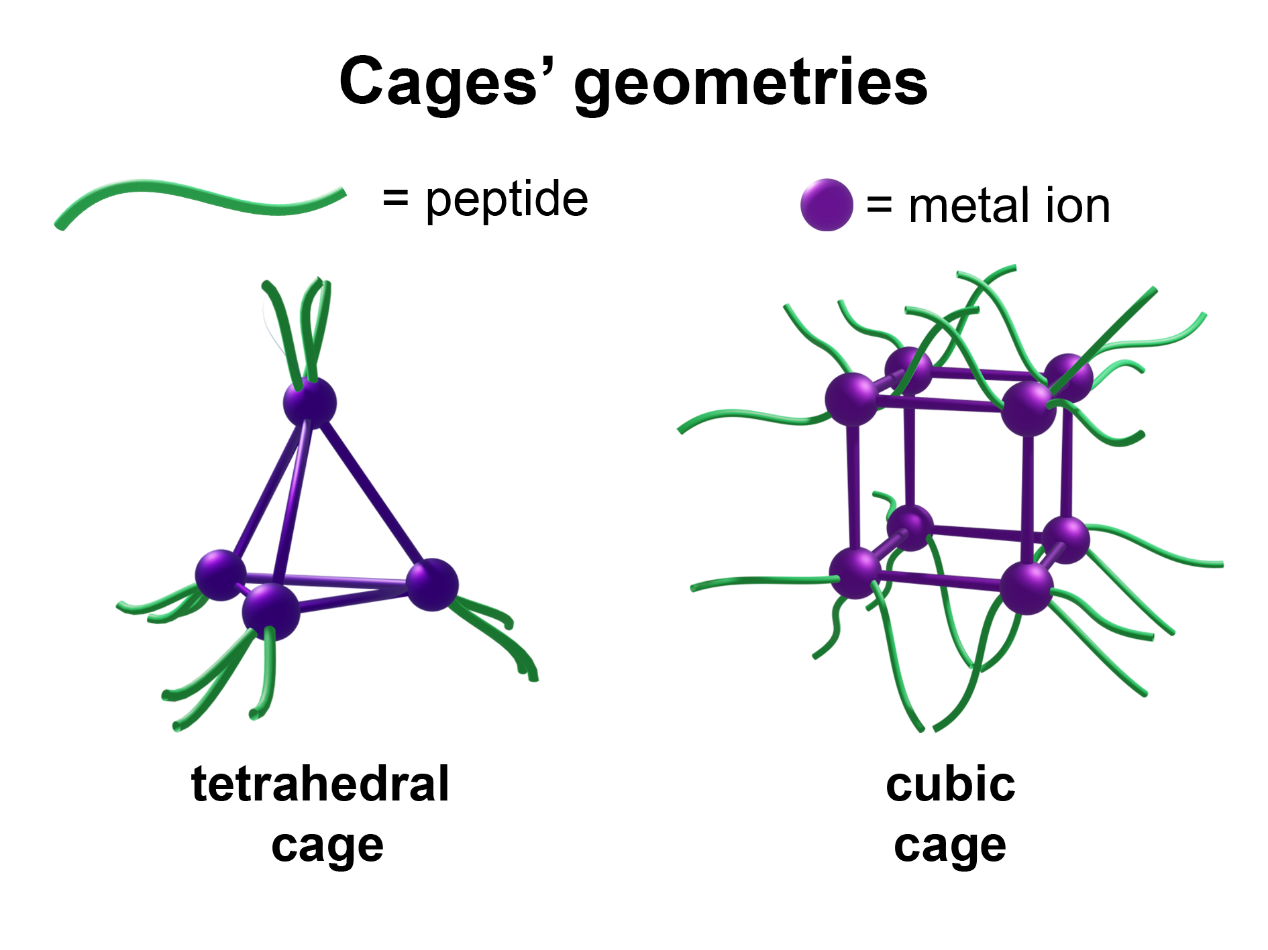
A bis-aldehyde allows the formation of a tetrahedral cage having the ligands on the edges of the tetrahedra and not on the faces. This difference allows to obtain a cage with bigger porous and it could have an influence in the subsequent gel. Regarding the second synthetised aldehyde, it can be oriented either clockwise or anticlockwise, and furthermore, each trischelated octahedral vertex of the tetrahedron may adopt either lambda or delta handedness.
Finally, the last aldehyde, containing four aldehydic group on the vertex, has been tested for the formation of a cage with different geometry, (a cube) than the previous two. This cubic cage possesses bigger porous that could encapsulate bigger guest as drugs or bioactive molecules, increasing the range of possible applications of the resulting gel.
The gels based on these different cages can help us to make more progress in understanding how their geometry and chirality can influence the topology and the properties of the supramolecular fibrils. The chemical, physical and mechanical will be studied using different techniques as rheology, electron microscopy, circular dichroism spectroscopy, but also advanced synchrotron radiation techniques as resonance Raman.
Dissemination
The STSM has resulted in a joint work and the manuscript of the project is preparation. Moreover, the Editor-in-Chief of Nature Nanotechnology expressed interest towards the preliminary data obtained before this STSM.