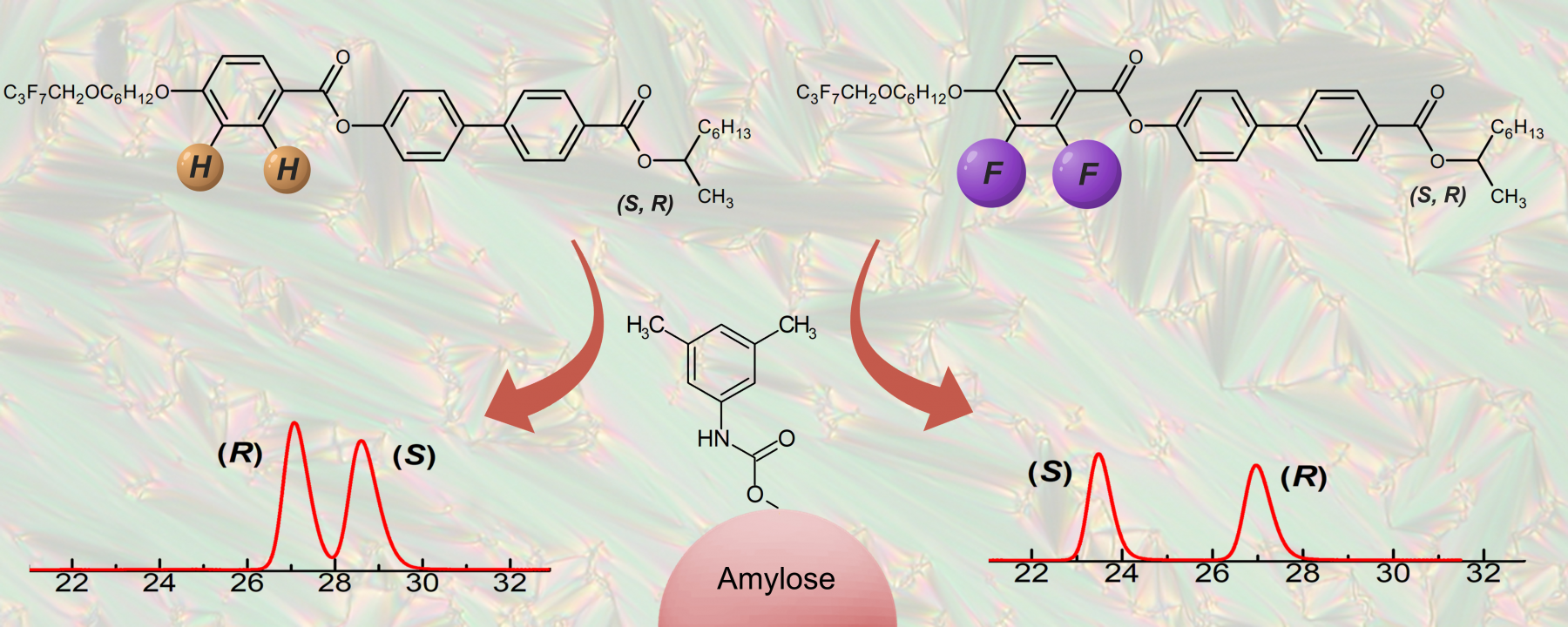
Currently, effective control of optical purity by chiral separation of (S) and (R) enantiomers remains a relevant and highlighted task when designing new chiral organic materials in general, especially for self-assembling materials possessing synclinic and anticlinic smectic phases. An efficient methodology for accomplishing this task was developed and verified with a series of chiral fluorinated liquid-crystalline materials with lateral substitution on the molecular core. The self-assembling behaviour of new racemic materials was established. Upon cooling from the isotropic phase, all materials possessed orthogonal, synclinic and anticlinic smectic mesophases. The materials were available with four fluorine substitution patterns and in racemic and pure (R) and (S) enantiomer forms. Chiral high-performance liquid chromatography was accomplished using polysaccharide-based chiral stationary phases. All separations were performed in normal chromatographic mode, and the baseline separation of all enantiomer pairs was achieved using chiral stationary phases based on derivatised amylose and cellulose. The enantiomer elution order for racemic mixtures was verified by comparing their retention times with those of the respective pure (R) and (S) enantiomers. Interestingly, two materials demonstrated an unexpected switch in enantiomer elution order. This is very important information that is useful, for instance, for potential utilisation in preparative scale chiral chromatography.
Download “Article preprint” Effective_control_optical_purity.pdf – Downloaded 246 times – 735 KB
Download a copy of the manuscript (preprint version)